KING OF PRUSSIA, PA – September 28, 2020 – CSL Behring, a global biotherapeutics leader, announced today that the U.S. Food and Drug Administration (FDA) has approved an expanded indication for Haegarda® (C1 Esterase Inhibitor Subcutaneous [Human]) for routine prophylaxis to prevent hereditary angioedema (HAE) attacks in patients 6 years of age and older. A rare, genetic and potentially life-threatening condition, HAE affects about 1 in 50,000 people in the United States1, causing painful, potentially debilitating and unpredictable episodes of swelling of the abdomen, larynx, face and extremities, among other areas of the body.2 Children have a 50% chance of inheriting HAE if one of their parents has the disease.2 Haegarda, the HAE therapy that reduces attacks by a median of 95%, is now the first and only subcutaneous treatment option for prevention of hereditary angioedema (HAE) attacks in patients 6 years of age and older. In addition to the expanded pediatric indication, the updated label now includes clinical safety data regarding Haegarda use in pregnant women.
“Since 2017, Haegarda has been a trusted and effective option for patients seeking to prevent HAE attacks, but until now preventative options for younger children living with the condition have been limited,” said Debra Bensen-Kennedy, MD, Vice President, North America Medical Affairs, CSL Behring. “With this expanded indication, we are able to offer pediatric patients as young as 6 years of age, an effective, preventative subcutaneous solution and deliver on our promise of addressing the unmet needs of people living with HAE.”
The latest FDA approval was based on results from two CSL Behring-sponsored COMPACT (Clinical Study for Optimal Management of Preventing Angioedema with Low-Volume Subcutaneous C1-Inhibitor Replacement Therapy) trials: COMPACT Pivotal Study and COMPACT Open Label Extension (OLE) Study. COMPACT, an international, prospective multi-center, randomized, double-blind, placebo-controlled Phase 3 pivotal study, included six subjects aged 17 years or younger with symptomatic HAE. In the COMPACT pivotal study, Haegarda, at the FDA approved dose of 60 IU/kg, reduced the number of HAE attacks by a median of 95% relative to placebo. Use of rescue medication was reduced by a median of greater than 99% versus placebo.3 COMPACT OLE featured 126 subjects, including nine patients ages 17 years or younger. In this trial, all nine pediatric subjects experienced greater than 50 percent reduction in number of attacks per month versus the pre-study period, with a median of 97% reduction in the median number of attacks/month (0.11).4 All subjects had less than one attack/4-week period and four had less than one attack/year (one subject was attack free).4,5 No subject discontinued treatment due to a treatment-related adverse event. Safety and effectiveness results of subgroup analysis by age was consistent with overall study results.
“The FDA’s approval of Haegarda for preventing HAE attacks in children six years of age and older represents a major stride in filling an unmet medical need. The U.S. Hereditary Angioedema Association is excited that there is now a subcutaneous prophylaxis option for the younger members of our HAE community and the families that care for them,” said Anthony J. Castaldo, U.S. HAEA President & Chief Executive Officer. The new label now includes results from the randomized, open-label, active treatment controlled study regarding four patients who became pregnant during the study, and received treatment until pregnancy was identified. These patients ranged in age from 19 to 32 and received C1-INH (S.C. administration). Patients received Haegarda for 4 – 8 weeks (9 – 15 doses) during the first trimester. As noted, these women reported no complications during delivery and all women delivered healthy babies.5, 6
“As the mother of a young patient and an active member of the HAE community, this news brings tears to my eyes as I am reminded of our struggles and strides over the years,” says Lisa Chacon, HAE patient advocate. “It warms my heart to know that so many children will now have access to this convenient, preventative therapy.”
CSL Behring is driven by its promise to save and improve the lives of HAE patients and their families and is committed to helping patients get access to its innovative therapies, regardless of their financial situation. For more information about Haegarda, including the U.S. Prescribing Information, visit www.HAEGARDA.com.
About Haegarda (C1 Esterase Inhibitor Subcutaneous [Human])
Haegarda is a self-administered, plasma-derived concentrate of C1-esterase inhibitor and the only subcutaneous therapy approved in the United States for routine prophylaxis to prevent HAE attack in patients 6 years of age and older. Haegarda targets the root cause of HAE by replacing deficient or dysfunctional C1-INH, restoring functional C1-INH levels to above 40 percent of normal levels, which is proposed to reduce the risk of HAE attacks. Haegarda is dosed individually based on body weight so that each patient can achieve functional C1-INH levels.
About Hereditary Angioedema
A rare, genetic and potentially life-threatening condition, HAE causes painful, debilitating and unpredictable episodes of swelling of the abdomen, larynx, face and extremities, among other areas of the body. HAE is one of two forms of bradykinin-mediated angioedema, the other being nonhereditary or acquired angioedema. HAE is caused by deficient or dysfunctional C1-INH, a key protein in the body that controls swelling. The defect with C1-INH lies within a person’s genetic code, which is why HAE runs in families. HAE is classified as either type I, type II or HAE with normal C1-INH levels.
About CSL Behring
CSL Behring is a global biotherapeutics leader driven by its promise to save lives. Focused on serving patients’ needs by using the latest technologies, we develop and deliver innovative therapies that are used to treat coagulation disorders, primary immune deficiencies, hereditary angioedema, respiratory disease, and neurological disorders. The company’s products are also used in cardiac surgery, burn treatment and to prevent hemolytic disease of the newborn.
CSL Behring operates one of the world’s largest plasma collection networks, CSL Plasma. The parent company, CSL Limited (ASX:CSL;USOTC:CSLLY), headquartered in Melbourne, Australia, employs more than 27,000 people, and delivers its life-saving therapies to people in more than 100 countries. For inspiring stories about the promise of biotechnology, visit Vita CSLBehring.com/vita and follow us on Twitter.com/CSLBehring.
1.Lumry WR. Am J Manag Care. 2013;19:S103-S110)
2.US Hereditary Angioedema Association. Retrieved on Aug 24, 2020, from https://www.haea.org/
3.COMPACT: Longhurst H, Cicardi M, Craig T, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. 2017;376:1131-40.
4.Manning M, Caballero T, Hussain I, et al. Long-term efficacy of subcutaneous C1-inhibitor in pediatric patients with hereditary angioedema. Poster presented at: 2018 Annual Scientific Meeting of the American College of Allergy, Asthma and Immunology; November 15-19, 2018; Seattle WA.
5.COMPACT OLE: Craig T, Zuraw B, Longhurst H, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2019;7(6):1793-1802.
6.Levy D, Farkas H, Reidel M, et al. Long-term efficacy and safety of subcutaneous C1-inhibitor in women with hereditary angioedema: subgroup analysis from an open-label extension of a Phase 3 trial. Allergy, Asthma Clin. Immunology; 2020; 16(8):doi: https://doi.org/10.1186/s13223-020-0409-3.
Source: CSL Behring
Posted: September 2020
Related Articles:
Haegarda (C1 esterase inhibitor (human)) FDA Approval History
Related Post
 26
26 Feb
The Cost of Loneliness in Retirement
While we frequently stress over saving enough for retirement, the expense of depression is ascending as an equivalent danger to a blissful, solid retirement. Yet, there are steps you can take now to fabricate your social capital. Depression and social confinement.
Read More 26
26 Feb
10 Ways to Reduce Your Risk of Colorectal Cancer
Colorectal malignant growth is the subsequent driving reason for disease related passings, influencing millions around the world. As per the American Malignant growth Society, around 1 out of 20 individuals will be determined to have colorectal disease during their lifetime..
Read More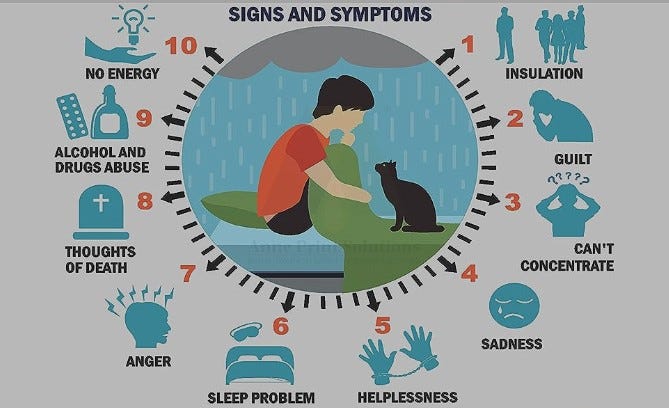 26
26 Feb
10 Signs You May Need Mental Health Treatment
Emotional well-being is really significant for our general wellbeing and prosperity. Psychological well-being influences the manner in which we think, feel, act, and affects others in our lives also. Psychological well-being can be affected by the climate, hereditary qualities, and.
Read More 26
26 Feb
10 Health Benefits of Eating Eggs for Breakfast
Might it be said that you are fed up with having exactly the same thing for breakfast consistently? Does your morning meal leave you feeling hungry simply a brief time frame after you eat? Then eating a couple of eggs.
Read More