KENILWORTH, N.J.–(BUSINESS WIRE) April 28, 2020 –Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has approved an additional recommended dosage of 400 mg every six weeks (Q6W) for Keytruda, Merck’s anti-PD-1 therapy, across all adult indications, including monotherapy and combination therapy. This indication is approved under accelerated approval based on pharmacokinetic data, the relationship of exposure to efficacy and the relationship of exposure to safety. Continued approval for this dosing may be contingent upon verification and description of clinical benefit in the confirmatory trials. This new dosage option will be available in addition to the current dose of 200 mg every three weeks (Q3W).
“The important social distancing measures for COVID-19 have created a number of challenges for people with cancer, including keeping to planned treatment schedules,” said Dr. Roy Baynes, senior vice president and head of global clinical development, chief medical officer, Merck Research Laboratories. “Today’s approval of an every six-week dosing schedule for Keytruda gives doctors an option to reduce how often patients are at the clinic for their treatment.”
Immune-mediated adverse reactions, which may be severe or fatal, can occur with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis and renal dysfunction, severe skin reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation (HSCT). Based on the severity of the adverse reaction, Keytruda should be withheld or discontinued and corticosteroids administered if appropriate. Keytruda can also cause severe or life-threatening infusion-related reactions. Based on its mechanism of action, Keytruda can cause fetal harm when administered to a pregnant woman. For more information, see “Selected Important Safety Information” below.
During this unprecedented time, Merck is committed to ensuring our medicines and vaccines reach our patients and customers. This includes a number of new steps to support patients in the U.S. who may have lost their jobs and insurance coverage. To learn more about resources Merck has available for patients who need help during the COVID-19 pandemic and beyond, visit our information page. For more detail about Merck’s response to the COVID-19 pandemic, please visit www.merck.com/COVID-19.
About Keytruda (pembrolizumab) Injection, 100 mg
Keytruda is an anti-PD-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. Keytruda is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells.
Merck has the industry’s largest immuno-oncology clinical research program. There are currently more than 1,200 trials studying Keytruda across a wide variety of cancers and treatment settings. The Keytruda clinical program seeks to understand the role of Keytruda across cancers and the factors that may predict a patient’s likelihood of benefitting from treatment with Keytruda, including exploring several different biomarkers.
Selected Keytruda (pembrolizumab) Indications
Melanoma
Keytruda is indicated for the treatment of patients with unresectable or metastatic melanoma.
Keytruda is indicated for the adjuvant treatment of patients with melanoma with involvement of lymph node(s) following complete resection.
Non-Small Cell Lung Cancer
Keytruda, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
Keytruda, in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, is indicated for the first-line treatment of patients with metastatic squamous NSCLC.
Keytruda, as a single agent, is indicated for the first-line treatment of patients with NSCLC expressing PD-L1 [tumor proportion score (TPS) ≥1%] as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and is stage III where patients are not candidates for surgical resection or definitive chemoradiation, or metastatic.
Keytruda, as a single agent, is indicated for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving Keytruda.
Small Cell Lung Cancer
Keytruda is indicated for the treatment of patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least 1 other prior line of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Head and Neck Squamous Cell Cancer
Keytruda, in combination with platinum and fluorouracil (FU), is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC).
Keytruda, as a single agent, is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [combined positive score (CPS) ≥1] as determined by an FDA-approved test.
Keytruda, as a single agent, is indicated for the treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum-containing chemotherapy.
Classical Hodgkin Lymphoma
Keytruda is indicated for the treatment of adult and pediatric patients with refractory classical Hodgkin lymphoma (cHL), or who have relapsed after 3 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Primary Mediastinal Large B-Cell Lymphoma
Keytruda is indicated for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after 2 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. KEYTRUDA is not recommended for treatment of patients with PMBCL who require urgent cytoreductive therapy.
Urothelial Carcinoma
Keytruda is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 [combined positive score (CPS) ≥10], as determined by an FDA-approved test, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Keytruda is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
Keytruda is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
Microsatellite Instability-High (MSI-H) Cancer
Keytruda is indicated for the treatment of adult and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options, or
colorectal cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials. The safety and effectiveness of Keytruda in pediatric patients with MSI-H central nervous system cancers have not been established.
Gastric Cancer
Keytruda is indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Esophageal Cancer
Keytruda is indicated for the treatment of patients with recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy.
Cervical Cancer
Keytruda is indicated for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Hepatocellular Carcinoma
Keytruda is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Merkel Cell Carcinoma
Keytruda is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC). This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Renal Cell Carcinoma
Keytruda, in combination with axitinib, is indicated for the first-line treatment of patients with advanced renal cell carcinoma (RCC).
Adult Indications: Additional Dosing Regimen of 400 mg Every 6 Weeks
Keytruda is indicated for use at an additional recommended dosage of 400 mg every 6 weeks for all approved adult indications. This indication is approved under accelerated approval based on pharmacokinetic data, the relationship of exposure to efficacy, and the relationship of exposure to safety. Continued approval for this dosing may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Merck’s Focus on Cancer
Our goal is to translate breakthrough science into innovative oncology medicines to help people with cancer worldwide. At Merck, the potential to bring new hope to people with cancer drives our purpose and supporting accessibility to our cancer medicines is our commitment. As part of our focus on cancer, Merck is committed to exploring the potential of immuno-oncology with one of the largest development programs in the industry across more than 30 tumor types. We also continue to strengthen our portfolio through strategic acquisitions and are prioritizing the development of several promising oncology candidates with the potential to improve the treatment of advanced cancers. For more information about our oncology clinical trials, visit www.merck.com/clinicaltrials.
About the Merck Access Program for KEYTRUDA
At Merck, we are committed to supporting accessibility to our cancer medicines. Merck provides multiple programs to help appropriate patients who are prescribed KEYTRUDA have access to our anti-PD-1 therapy. The Merck Access Program provides reimbursement support for patients receiving KEYTRUDA, including information to help with out-of-pocket costs and co-pay assistance for eligible patients. More information is available by calling 855-257-3932 or visiting www.merckaccessprogram-keytruda.com.
About Merck’s Patient Support Program for KEYTRUDA
Merck is committed to helping provide patients and their caregivers support throughout their treatment with KEYTRUDA. The KEY+YOU Patient Support Program provides a range of resources and support. For further information and to sign up, eligible patients may call 85-KEYTRUDA (855-398-7832) or visit www.keytruda.com.
About Merck
For more than 125 years, Merck, known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world’s most challenging diseases in pursuit of our mission to save and improve lives. We demonstrate our commitment to patients and population health by increasing access to health care through far-reaching policies, programs and partnerships. Today, Merck continues to be at the forefront of research to prevent and treat diseases that threaten people and animals – including cancer, infectious diseases such as HIV and Ebola, and emerging animal diseases – as we aspire to be the premier research-intensive biopharmaceutical company in the world. For more information, visit www.merck.com and connect with us on Twitter, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Kenilworth, N.J., USA
This news release of Merck & Co., Inc., Kenilworth, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the recent global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s 2019 Annual Report on Form 10-K and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
Source: Merck
Posted: April 2020
Related Articles:
- FDA Approves Keytruda (pembrolizumab) for Patients With BCG-Unresponsive, High-Risk, Non-Muscle Invasive Bladder Cancer – January 8, 2020
- FDA Approves Keytruda (pembrolizumab) plus Lenvima (lenvatinib) Combination Treatment for Patients with Certain Types of Endometrial Carcinoma – September 17, 2019
- FDA Approves Keytruda (pembrolizumab) for Recurrent Locally Advanced or Metastatic Squamous Cell Carcinoma of the Esophagus – July 31, 2019
- FDA Approves Keytruda (pembrolizumab) for the Treatment of Metastatic Small Cell Lung Cancer (SCLC) – June 18, 2019
- FDA Approves Keytruda (pembrolizumab) for First-Line Treatment of Head and Neck Squamous Cell Carcinoma – June 11, 2019
- FDA Approves Keytruda (pembrolizumab) in Combination With Inlyta (axitinib) as First-Line Treatment for Patients With Advanced Renal Cell Carcinoma (RCC) – April 22, 2019
- FDA Approves Expanded Monotherapy Label for Merck’s Keytruda (pembrolizumab) for First-Line Treatment of NSCLC – April 11, 2019
- FDA Approves Keytruda (pembrolizumab) for the Adjuvant Treatment of Patients with Melanoma with Involvement of Lymph Node(s) Following Complete Resection – February 19, 2019
- FDA Approves Keytruda (pembrolizumab) for the Treatment of Patients with Recurrent Locally Advanced or Metastatic Merkel Cell Carcinoma – December 19, 2018
- FDA Approves Keytruda (pembrolizumab) for the Treatment of Patients with Hepatocellular Carcinoma (HCC) Who Have Been Previously Treated with Sorafenib – November 9, 2018
- FDA Approves Keytruda (pembrolizumab) in Combination with Carboplatin and Either Paclitaxel or Nab-Paclitaxel for the First-Line Treatment of Patients with Metastatic Squamous Non-Small Cell Lung Cancer (NSCLC) – October 30, 2018
- FDA Approves Expanded Label for Merck’s Keytruda (pembrolizumab) in Patients with Metastatic Nonsquamous NSCLC with No EGFR or ALK Genomic Tumor Aberrations – August 21, 2018
- FDA Approves Keytruda (pembrolizumab) for Treatment of Refractory or Relapsed Primary Mediastinal Large B-Cell Lymphoma (PMBCL) – June 13, 2018
- FDA Approves Keytruda (pembrolizumab) for Previously Treated Patients with Recurrent or Metastatic Cervical Cancer Whose Tumors Express PD-L1 – June 12, 2018
- FDA Approves Merck’s Keytruda (pembrolizumab) for Previously Treated Patients with Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer Whose Tumors Express PD-L1 – September 22, 2017
- FDA Approves Keytruda (pembrolizumab) as First Cancer Treatment for any Solid Tumor with a Specific Genetic Feature – May 23, 2017
- FDA Approves Merck’s Keytruda (pembrolizumab) for Certain Patients with Locally Advanced or Metastatic Urothelial Carcinoma – May 18, 2017
- FDA Approves Merck’s Keytruda (pembrolizumab) as First-Line Combination Therapy for Patients with Metastatic Nonsquamous Non-Small Cell Lung Cancer (NSCLC), Irrespective of PD-L1 Expression – May 10, 2017
- FDA Approves Merck’s Keytruda (pembrolizumab) for Classical Hodgkin Lymphoma (cHL) – March 15, 2017
- FDA Approves Merck’s Keytruda (pembrolizumab) for First-Line Treatment of Certain Patients with Metastatic Non-Small Cell Lung Cancer – October 24, 2016
- FDA Approves Merck’s Keytruda (pembrolizumab) for Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma – August 5, 2016
- FDA Approves Expanded Indication for Keytruda (pembrolizumab) for the Treatment of Patients with Advanced Melanoma – December 18, 2015
- FDA Approves Keytruda (pembrolizumab) for Advanced Non-Small Cell Lung Cancer – October 2, 2015
- FDA Approves Keytruda (pembrolizumab) for Advanced Melanoma – September 4, 2014
- Merck to Present New Data in Five Tumor Types from Studies Evaluating Pembrolizumab – September 2, 2014
- Merck’s Investigational Anti-PD-1 Antibody, Pembrolizumab, Under Regulatory Review in Europe for Advanced Melanoma – June 30, 2014
Keytruda (pembrolizumab) FDA Approval History
Related Post
 26
26 Feb
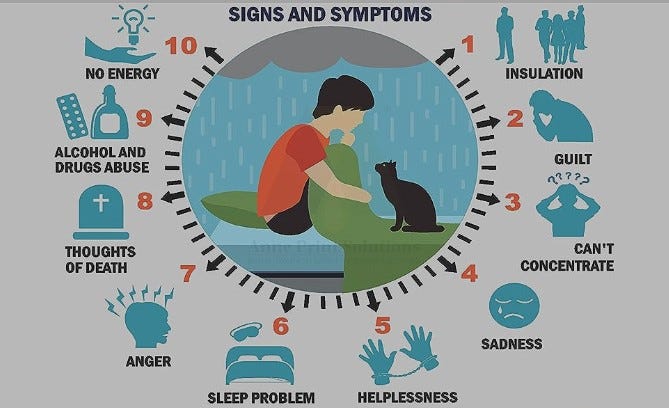
10 Signs You May Need Mental Health Treatment
Emotional well-being is really significant for our general wellbeing and prosperity. Psychological well-being influences the manner in which we think, feel, act, and affects others in our lives also. Psychological well-being can be affected by the climate, hereditary qualities, and.
Read More 26
26 Feb
10 Health Benefits of Eating Eggs for Breakfast
Might it be said that you are fed up with having exactly the same thing for breakfast consistently? Does your morning meal leave you feeling hungry simply a brief time frame after you eat? Then eating a couple of eggs.
Read More 26
26 Feb
Study Reveals Extent of Undiagnosed Cancer Cases Due to COVID-19 Pandemic
North of 134,000 disease cases went undiscovered in the U.S. during the initial 10 months of the Coronavirus pandemic, as per another College of Kentucky Markey Disease Center review. The report distributed in JAMA Oncology gives the principal assessments of.
Read More 26
26 Feb
Another COVID Toll: Over 134,000 Undiagnosed Cancers During Height of Pandemic
In excess of 134,000 diseases could have gone undiscovered during the initial 10 months of the Coronavirus pandemic, as per an investigation of public patterns in malignant growth rate. Yearly disease occurrence fell practically 30% shy of the normal rate from.
Read More